DieselNet Technology Guide » Hydrogen Fueled Engines
DieselNet | Copyright © ECOpoint Inc. | Revision 2023.12
This is a preview of the paper, limited to some initial content. Full access requires DieselNet subscription.
Please log in to view the complete version of this paper.
As energy systems de-fossilize and low carbon electricity production increases, hydrogen is promoted as an energy storage medium that could be used during periods of excess electricity production. In this context, the prospects of hydrogen fueled internal combustion engines (hydrogen ICE) as a transport decarbonization pathway are increasingly being explored, and they are thus the subject of active research and development. There is particular interest from the heavy-duty and nonroad sectors where powertrain electrification is challenging because of high power density and “range” requirements [5982][5800]. From a technical feasibility standpoint, the hydrogen ICE has been demonstrated as a viable technology (e.g., Ford [5984], BMW [5985], JCB [5800], Mazda [5986]) but for it to be competitive against other decarbonization options, most notably electrification and hydrogen fuel cells, its efficiency and power density need to be improved and low pollutant emissions (mainly NOx) need to be ensured. There are limitations to such improvements for port fuel-injected (PFI) hydrogen engines, which can potentially be addressed by direct-injected (DI) engine designs. This article presents an overview of the direct-injected hydrogen engine concept and discusses the potential of different injection strategies and their current state of development.
Hydrogen has unique thermochemical properties that offer both opportunities and challenges for engine development [5982][5988][5987]. A summary of these properties and their impact on engine performance relative to gasoline engines is provided in Table 1. For the topic at hand (injection and mixture formation), relevant properties of hydrogen are its:
Moreover, hydrogen has a higher ratio of specific heats, thermal conductivity, and speed of sound than comparable natural gas and gasoline mixtures [5982].
| Property | Comparison with Gasoline | Positive Effects | Negative Effects |
|---|---|---|---|
| Density | Lower (0.08 vs 692 kg/m3 at 300 K and 1 bar [5982] |
|
|
| Volumetric energy density | Lower (10.7 vs 33×103 MJ/m3 at 1 bar and 273 K [5987] |
| |
| Heating value | Higher (LHV: 120 vs 44.3 MJ/kg [5982]) |
| |
| Laminar Flame Speedb | Higher (185 vs 40 cm/s at φ = 1, 1 bar, 298 K) |
|
|
| Minimum ignition energy | Lower (0.02 vs 0.28 mJ at 300 K, 1 bar [5982]) |
|
|
| Quench distance | Shorter (0.64 vs 2.84 mm at φ = 1 [5988]) |
|
|
| Flammability Limits | Wider (φ = 0.1-7 vs 0.66-3.85 at 300 K, 1 barc [5982]) |
|
|
| Mass diffusivity (in air) | Higher (0.61 vs 0.07 cm2/s at 1 bar and 300 K [5982]) |
|
|
| Auto-ignitability | Unclear (higher RON, lower MON, higher AI temperature, low methane number)d |
|
|
| a The Power deficit of a stoichiometric hydrogen PFI engine with 29% lower volumetric efficiency than a comparable gasoline engine decreased to 15% because of the higher LHV [5984]. b The suitability of using LFS as a flame speed index for hydrogen is unclear because of the rapid onset of cellularity, which increases the actual flame speed and makes the determination of LFS difficult. Limited LFS data is available at engine-relevant conditions [5987]. c Flammability limits widen with temperature and pressure. For engine-relevant conditions, practical lean limits are around φ = 0.25. d Ambiguity exists about the relative auto-ignitability of hydrogen and the suitability of using existing auto-ignition indices. High RON and low MON values of 130 and 60-65, respectively, have been reported for hydrogen. The low MON values are a consequence of hydrogen’s low minimum ignition energy, which at the high-temperature MON conditions leads to knocking. On the methane number scale, hydrogen has a value of 0 which suggests that it has a very high propensity to knock compared to other gaseous fuels. The auto-ignition temperature of hydrogen, 585°C, is higher than diesel (> 225°C) and gasoline (230-450°C) [5991]. | |||
Modern hydrogen engines need to simultaneously meet multiple operational requirements. These include:
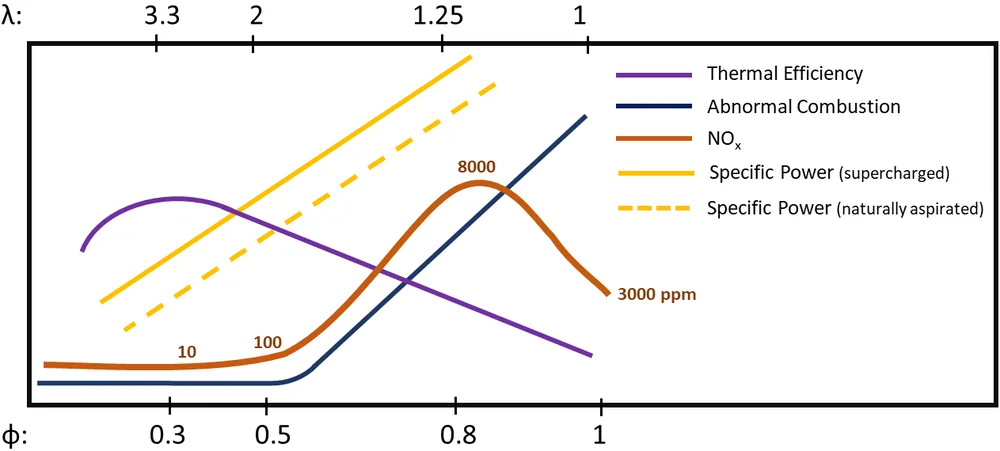
Figure 1 qualitatively illustrates the trade-offs between these operational requirements. The combustion strategy employed to meet these requirements determines an engine’s mixture preparation needs. It defines the operating equivalence ratio (φ) and in turn the amount of hydrogen and air that need to be supplied, the injection system type, timing of hydrogen supply, and the nature of the combustion mixture formed (i.e., homogeneous or stratified).
As illustrated in Figure 1, lean combustion (φ < 0.5) can abate NOx emissions and thus minimize the need for NOx aftertreatment. However, engine power is reduced as less chemical energy (from the fuel) is available. Supercharging can help mitigate this. NOx emissions increase rapidly at φ > 0.5 and peak around φ = 0.75. Ultra lean operation (φ < 0.3) is undesirable from an engine efficiency perspective as flame speed decreases and combustion slows down, adversely affecting combustion phasing and combustion efficiency.
Lean operation has the additional benefit of reducing the frequency of abnormal combustion events because the minimum ignition energy of hydrogen-air mixtures increases rapidly when φ is decreased from stoichiometric [5992].
Stoichiometric operation is desirable from a power output perspective even though it increases NOx emissions and the probability of abnormal combustion. Stoichiometric combustion does, however, permit the use of NOx reduction catalysts (similar to three-way catalysts) whose conversion efficiency drops significantly and rapidly at lean conditions. For moderately lean mixtures (0.5 < φ < 1) selective catalytic reduction (SCR) can be used.
Figure 1 presents the general behavior for well-mixed hydrogen-air mixtures. For stratified mixtures that have a range of φ, the cumulative effects of the combustion of different φ strata would be witnessed. For example, in a universally lean but stratified mixture, regions with φ > 0.5 will produce high NOx emissions.

###